New DF and Quality Officers join the FCN
30th September 2024
In order to meet part of the requirements for ISO 15189 accreditation, SARCs will need to demonstrate that the consumables, that are critical to the recovery of DNA evidence, used during a forensic medical examination are manufactured to the ISO 18385 Forensic DNA Grade Standard (the scope of this standard is minimising the risk of human DNA contamination in products used to collect, store and analyse biological material for forensic purposes) and continue to meet this standard through the SARC receipt and storage process, up to the point of use.
To address this requirement the FCN are coordinating a SARC National Consumable Validation in collaboration with SceneSafe and Cellmark Forensic Services. Every SARC in England and Wales has signed up to participate, with estimated savings of £0.5M.
Forensic Science Regulator Gary Pugh said:
“The quality assurance of materials used for collecting DNA in Sexual Assault Referral Centre’s is critical to the accuracy and reliability of forensic evidence. I applaud the collaborative approach taken here, which is both operationally effective and value for money.”
This project involves all SARCs adapting their consumable receipt and storage process to be in line with the ISO 15189 standard and FSR requirements. SARCs are training their personnel in this procedure and updating their consumable storage ensuring that only forensic DNA grade consumables are used in the recovery of forensic evidence. Significant progress has already been made in the SARCs journey towards achieving accreditation and improving their service.
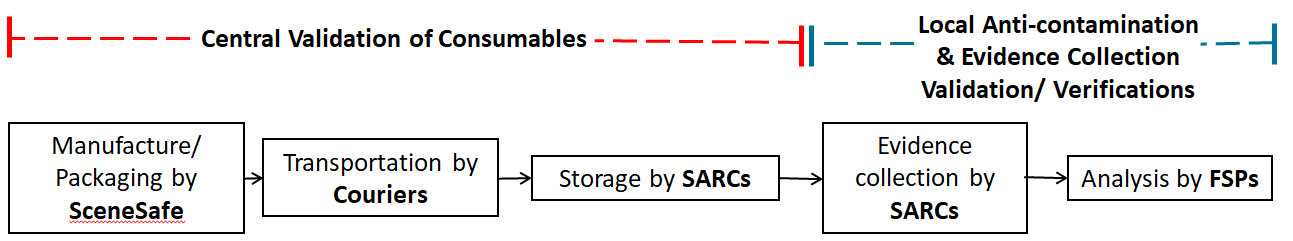
The FCN are coordinating the collection of a representative sample number of every forensic DNA grade consumable used by SARCs nationally. In the week commencing 24th January all the SARCs will be supplied with a box, packaging and instructions to provide 5 forensic DNA grade consumables from their consumable store which will be collected via courier and delivered to Cellmark Forensic Services for testing. Cellmark Forensic Services will report levels of DNA detected, results will be evaluated, and a validation report developed and issued by the FCN. The aim is for this validation report to provide the data required by every SARC in England and Wales to evidence that the consumables they are purchasing, plus their procedures for receipt and storage of consumables are fit for purpose in their SARC facility and carried out by their personnel.
More information is available on the FCN | Sexual Assault Referral Centre (SARC) Accreditation Support platform on Knowledge Hub.
For any queries, please contact Michelle Gaskell (Quality Specialist) National SARC Accreditation Lead: [email protected]